Quality Manual
Documents the company‘s operational procedures and processes with useful cross-references to GDPR, ISO or other standards, laws and regulations.
The Quality Manual provides an intuitive and user-friendly interface which makes the system easy to navigate and facilitates the registration of the company’s business operations and procedures. Documents are version controlled which ensures traceability and allows the user to keep track of changes in content. Every document goes through an inbuilt approval process and it’s possible to make cross-references to GDPR, ISO or other standards, laws and regulations that need compliance.
Features

Personalized dashboard
Gathers processes suitable for your role within the organization. Read them in your computer, tablet or smartphone.

All information in one place
Standard operation procedures, work-flow, work-descriptions, checklists and instructions are stored in one place

Confirm reading on published document
In the lead to secure validation of knowledge, the system provides a feature to confirm reading so managers know when employees have read and are familiar with new and important information.

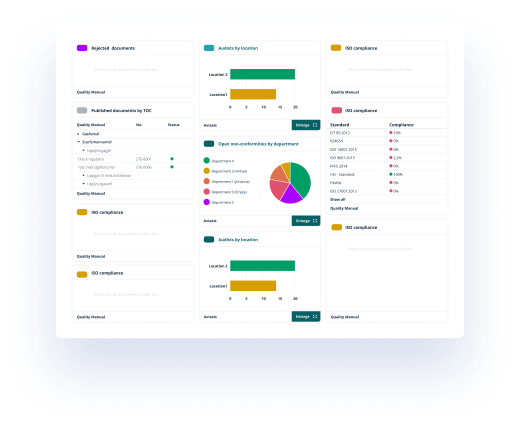
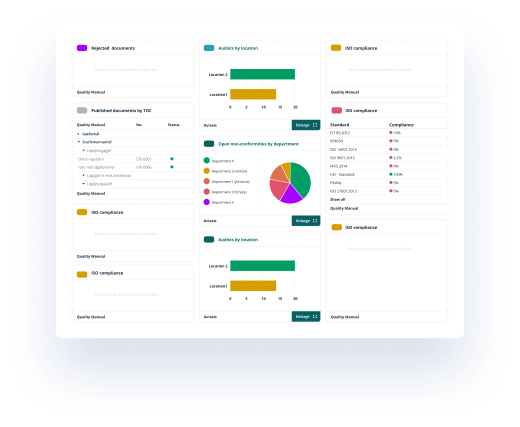
Categorization and trace-ability
All published documents are secured in the closed system according to FDA 21.CFR. Part 11, GMP, GL-44 and more, and the different views help employees, managers and auditors to control compliance
CCQ STUNDIN
GDPR is easy in CCQ
Work Book
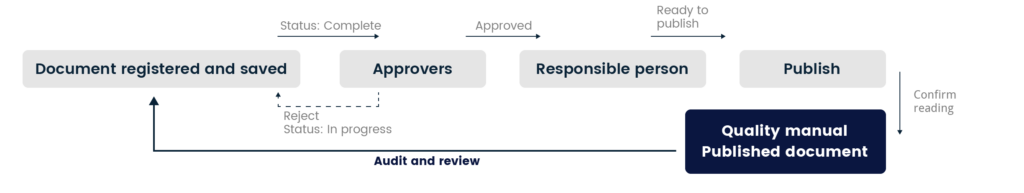
All documents are prepared and edited in the workbook of the Quality Manual. When a document is completed, it goes through a predefined approval process where it‘s reviewed and verified before it‘s finally is published. System contains strict trace ability and is version controlled.
If there are any issues to be found with the document before publication, every member involved approval process can reject the document and send it back to the editing stage.
The editable version contains different categorization options such as table of content, process, document type and access control. Review date is set and all document has its own version history. In the different views managers can follow status of the document and to whom.
Published document
When a document is published, a non-editable version appears in the Published documents interface. When a published document is up for review, the system sends the editors an email reminding them that the time has come to revise the document.
The workbook version of the published document is then re-evaluated, and the necessary adjustments are made. When the document is completed, the approval process starts all over again.
It is easy to search for a specific document. There is an inbuilt filter, search engine and the different views such as document type, reference to standard and my favourites guides you to correct document.
Work Book
All documents are prepared and edited in the workbook of the Quality Manual. When a document is completed, it goes through a predefined approval process where it‘s reviewed and verified before it‘s finally is published. System contains strict trace ability and is version controlled.
If there are any issues to be found with the document before publication, every member involved approval process can reject the document and send it back to the editing stage.
The editable version contains different categorization options such as table of content, process, document type and access control. Review date is set and all document has its own version history. In the different views managers can follow status of the document and to whom.

Published document
When a document is published, a non-editable version appears in the Published documents interface. When a published document is up for review, the system sends the editors an email reminding them that the time has come to revise the document.
The workbook version of the published document is then re-evaluated, and the necessary adjustments are made. When the document is completed, the approval process starts all over again.
It is easy to search for a specific document. There is an inbuilt filter, search engine and the different views such as document type, reference to standard and my favourites guides you to correct document.

